The focus was to develop a hemorrhoids treatment formulation based upon natural ingredients that would support and therefore help optimise the body’s own particular healing mechanisms that naturally combat the symptoms associated with haemorrhoids. It was decided to take a new and unique approach that could offer significant and immediate protective ability against the bacterial infections in the rectal area, irrespective of the stage of the disease process because these were known to be a major cause of such inflammations and other discomforts. It was determined that such a formulation should;
1. Allow safe and unlimited duration of use.
2. Have no side effects.
3. Be able to be used by pregnant and postnatal women.
4. Be suitable for use by both men and women of all ages, without contradiction to other medications or the risk of arousing inter-medicinal reactions.
The research and development involved three phases of clinical trials and over 200 patients of all ages who collectively presented all the various stages of haemorrhoid symptoms. Most had undergone previous treatments that included medical ointments, suppositories, gels, electrotherapy, ligation with elastic bands, sclerotherapy, laser or infrared cauterization and haemorrhoidectomy.
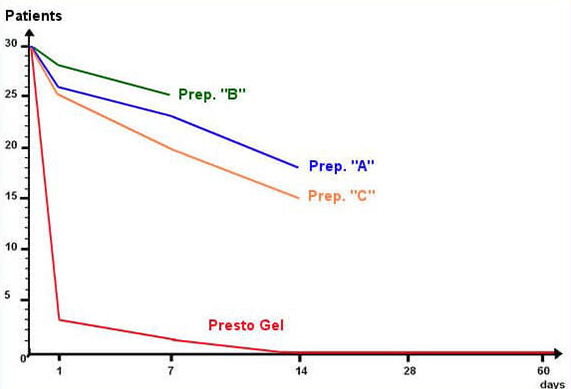
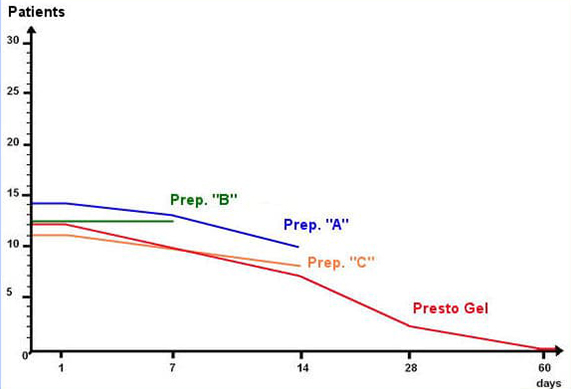
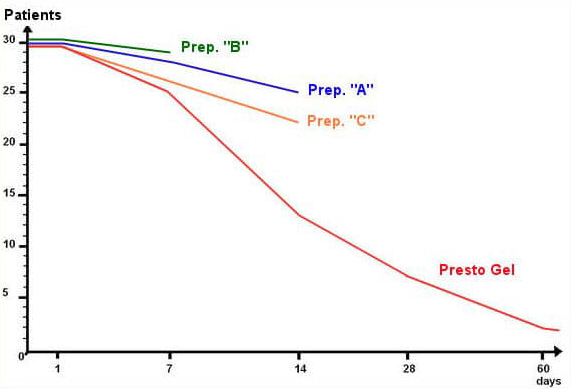
A unique method was developed to extract the selected active herbal ingredients without impairing their activity and clinical trials continued in three phases for fifteen months from February 2006 to April 2007 at the Israpharm Clinic in Israel until a formulation was achieved that showed significant clinical improvements over the performance of three representative haemorrhoid preparations (designated “A”, “B”, and “C” in the table below) containing combinations of the following standard ingredients: Ethyl-three-O-benzyl-D-glucofuroluzyl 3,5,6, Benzocaine, Hydrocortisone, Nifidipine and Zinc.
During the course of development, several discoveries were made that allowed the researchers to fully exploit and further enhance the latent activity of the selected ingredients. This potentiation plays a vital role in the relief that Presto Gel was observed to offer.
Once the formula had been optimised, Presto Gel was brought to market. Since then, it has been successfully used by many thousands of sufferers and although it is not a direct cure for hemorrhoids (there being no such thing), it is no exaggeration to say that today, Presto Gel has become as synonymous to haemorrhoids, as aspirin is to pain relief.